TMS for neuropsychiatric disorders
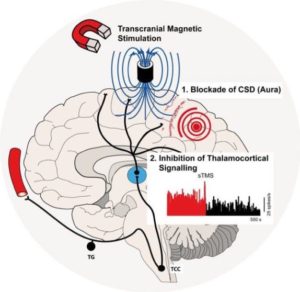 Repetitive transcranial magnetic stimulation (rTMS) it is one of the latest therapeutic methods for the treatment of various diseases and neuropsychiatric disorders, among which: depression, anxiety, attention deficit, schizophrenia, post-traumatic stress disorder, obsessive–compulsive and bipolar disorder, tinnitus, neuropathic pain, migraine, stroke , epilepsy, Parkinson’s disease. rTMS is a non-invasive treatment that delivers repetitive pulses of an MRI-strength magnetic field from a coil placed over the scalp. Powered by a rapidly pulsed current, the magnetic field passes unimpeded through the skull and stimulates brain tissue beneath, inducing currents that may help normalize activity in the area stimulated without producing seizure activity.
Repetitive transcranial magnetic stimulation (rTMS) it is one of the latest therapeutic methods for the treatment of various diseases and neuropsychiatric disorders, among which: depression, anxiety, attention deficit, schizophrenia, post-traumatic stress disorder, obsessive–compulsive and bipolar disorder, tinnitus, neuropathic pain, migraine, stroke , epilepsy, Parkinson’s disease. rTMS is a non-invasive treatment that delivers repetitive pulses of an MRI-strength magnetic field from a coil placed over the scalp. Powered by a rapidly pulsed current, the magnetic field passes unimpeded through the skull and stimulates brain tissue beneath, inducing currents that may help normalize activity in the area stimulated without producing seizure activity.
The FDA approved rTMS in 2008 as a treatment to alleviate symptoms of mildly treatment-resistant depression, in which patients have not found relief from antidepressant medication. It has also been studied as a possible treatment for a number of other disorders, such as schizophrenia, pain, stroke, and amyotrophic lateral sclerosis (ALS).
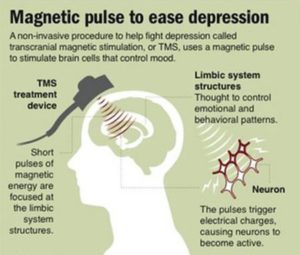
Antidepressant medications and psychotherapy are the first line treatments for major depression. These treatments, however, do not work for all patients. In these instances, rTMS might be used as an alternative treatment, or to augment antidepressant medications or psychotherapy. Patients who have failed to achieve an adequate response from antidepressants, or who are unable to tolerate medications, might consider rTMS therapy.
TMS was introduced in 1985, based on technology for experimentally probing brain activity. TMS was initially hoped to offer similar benefits to electroconvulsive therapy (ECT), without its drawbacks, such as impacting memory. ECT is still offered to patients who do not benefit from antidepressant medication, and is carried out under general anesthesia to limit discomfort from the convulsions it induces. By contrast, rTMS is carried out while the patient is awake and reclining in a specially equipped chair.
Sessions usually last 20-40 minutes, five days a week, typically for six weeks.
In open-label clinical trials, after 4-6 weeks of treatment, one out of two patients treated with rTMS for depression experienced a reduction in symptoms of 50% or more, and one out of three experienced remission. The effect was lower in patients who had exhibited resistance to more antidepressants.
The persistence of the effect is still being investigated. The therapeutic effect has been reported to last for at least six months, with intermittent repeat sessions as an option to prevent relapse. After coming in for daily treatments during the initial treatment phase, patients should continue to be monitored and receive maintenance therapy if needed, which may include receiving a medication.
While clinical trials indicate TMS is generally safe and well-tolerated. Due to its non-invasive nature and minimal risk of lasting side effects, rTMS has been studied as a possible treatment for a wide range of psychiatric conditions. The data are strongest for use in treatment of unipolar major depressive disorder. In schizophrenia, it has been under investigation to reduce the likelihood of hearing nonexistent voices (auditory hallucination) and of negative disease symptoms, such as apathy. It has also been studied for relieving symptoms of Parkinson’s disease, dystonia, tinnitus, anxiety, migraine, eating disorders and bipolar disorders, as well as for pain, stroke and ALS, among other conditions.